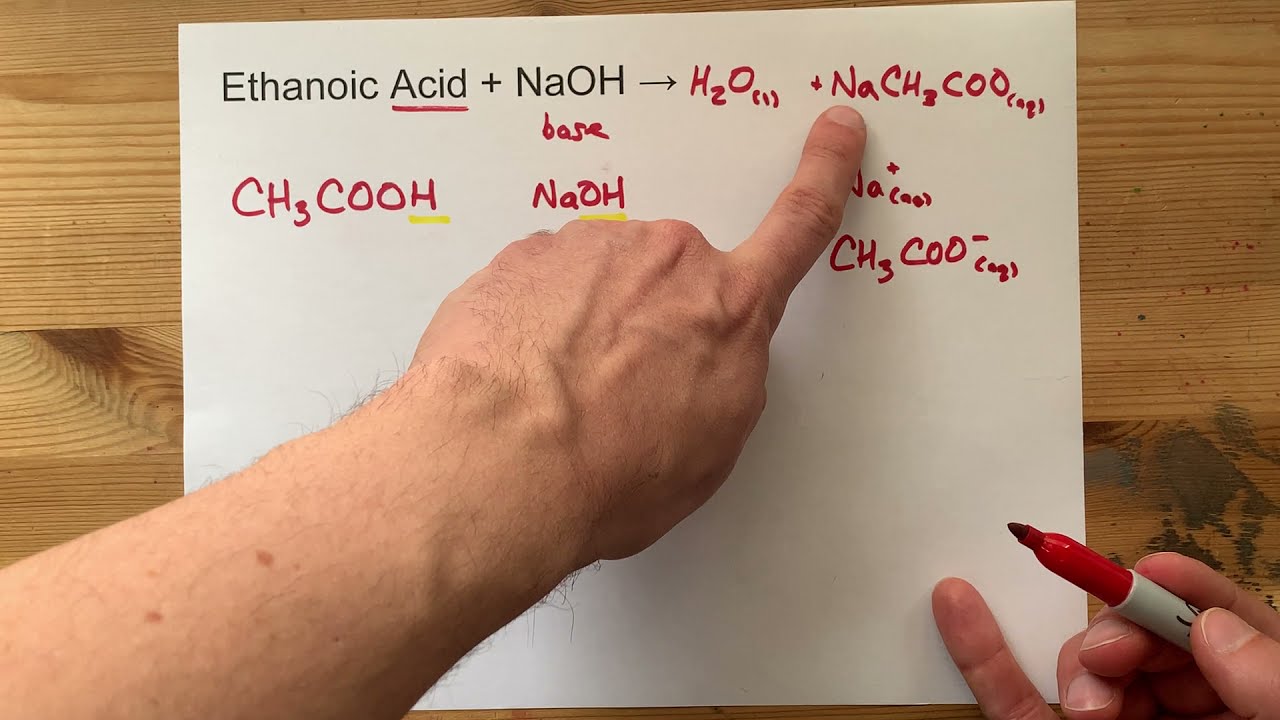
Ethanoic Acid and Sodium Hydroxide
There are two big advantages of doing this rather than using a dilute acid. Sour milk Lactic acid.

Net Ionic Equation For Naoh Ch3cooh Strong Base And Weak Acid
Includes kit list and safety instructions.

. 94-36-0 is a colorless rhombic crystalline solid. Amphoteric characteristics of chromium compounds. PH-dependent plant pigments that can be used as pH indicators occur in many plants including hibiscus red cabbage anthocyanin and grapes The juice of citrus fruits is acidic mainly because it contains citric acidOther carboxylic acids occur in many living systems.
A heterogeneous catalyst is used frequently in industry where gases flow through a solid catalyst which is often in the form of small pellets to increase the surface areaIt is often described as a fixed bed of catalyst Figure 5. The C 2H 4O. Sodium phenoxide is formed when phenol is treated with sodium hydroxide.
Aspirin acetylsalicylic acid is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Ii Reimer Tiemann reaction. It is prepared by reaction of benzoyl chloride sodium hydroxide and hydrogen peroxide.
A titration was performed to determine the concentration of an ethanoic acid C 2H 4O 2 solution using the following procedure. For example lactic acid is produced by muscle activity. For example sodium hydroxide NaOH aq in its aqueous solutions dissociates as.
Create bubbles fog and a colour change adding dry ice to alkaline ammonia or sodium hydroxide solution in this demonstration. Methanal and ethanoic acid in this demonstration. Vinegar is at least 4 acetic acid by volume making acetic acid the main component of vinegar apart from water and other trace elements.
B Fixed bed reactors. A few drops of indicator were added to the flask. Taking the same esters as above but using sodium hydroxide solution rather than a dilute acid.
Includes kit list and safety instructions. Tamarind T artaric acid 4. Acetic acid ə ˈ s iː t ɪ k systematically named ethanoic acid ˌ ɛ θ ə ˈ n oʊ ɪ k is an acidic colourless liquid and organic compound with the chemical formula CH 3 COOH also written as CH 3 CO 2 H C 2 H 4 O 2 or HC 2 H 3 O 2.
Ortho hydroxybenzoic acid as the main product when sodium phenoxide is treated with carbon dioxide followed by acidification it undergoes electrophilic substitution. Tubular reactors are used for example in the steam cracking of ethane propane and butane and naphtha to produce alkenes. One way of getting this for example would be to mix together 10 cm 3 of 10 mol dm-3 sodium ethanoate solution with 20 cm 3 of 10 mol dm-3 ethanoic acid.
Structure of Aspirin acetylsalicylic. Decorative gloss paints typically contain alkyd polymers resins. Or 10 cm 3 of 10 mol dm-3 sodium ethanoate solution with 10 cm 3 of 20 mol dm-3 ethanoic acid.
Aspirin can be prepared by reacting salicylic acid and acetic anhydride in the presence of an acid catalyst. The ester is heated under reflux with a dilute alkali like sodium hydroxide solution. B The ingredient meets the specifications of the Food Chemicals Codex 3d Ed.
35 which is incorporated by reference. The reactions are one-way rather than reversible and the products are easier to separate. A burette was filled with standard sodium hydroxide NaOH solution.
By using a solution with a known molarity and a colour indicator we measure how much of the solution is required to neutralise the unknown solution indicated by a change in the indicator which we. 2500 mL of the C 2H 4O 2 solution was pipetted into a conical flask. Acids Bases and Salts 158 SCIENCE AND TECHNOLOGY Notes MODULE -.
Acid base titration calculations help you identify properties such as pH of a solution during an experiment or what an unknown solution is when doing fieldwork. In other words the concentration of the ethanoate has to be half that of the ethanoic acid. A typical resin is that produced from a polyol such as propane-123-triol glycerol with a dibasic acid such as benzene-12-dicarboxylic phthalic anhydride and a drying oil linseed or soybean oil.
A Benzoyl peroxide C6H5CO2O2 CAS Reg. This reaction is known as Kolbes reaction. The state of protonation of phosphate derivatives.
Vinegar Ethanoic acid commonly called acetic acid 3. Some 3d metal compounds such as chromium hydroxide chromiumIII oxide ferric oxide has amphoteric characteristics. On being heated together ester linkages are formed and water is a by-product.
Amphoteric properties of chromium hydroxide CrOH 3 Chromium hydroxide CrOH 3 is an amphoteric compound and a green precipitateWhen NaOH aq is added that precipitate. Aspirin is a weak acid that is only slightly soluble in water.

How To Balance Naoh Ch3cooh Ch3coona H2o Youtube
What Is The Balanced Equation Between Sodium Hydroxide And Acetic Acid Quora

Naoh Ch3cooh Or Hc2h3o2 Nac2h3o2 H2o Balanced Equation Acetic Acid Or Ethanoic Acid Naoh Balance Youtube
0 Response to "Ethanoic Acid and Sodium Hydroxide"
Post a Comment